GAP ANALYSIS IN PHARMACEUTICALS / CHEMICALS / FMCG QUALITY /MANUFACTURING PROCESS AND ENGINEERING FACILITY
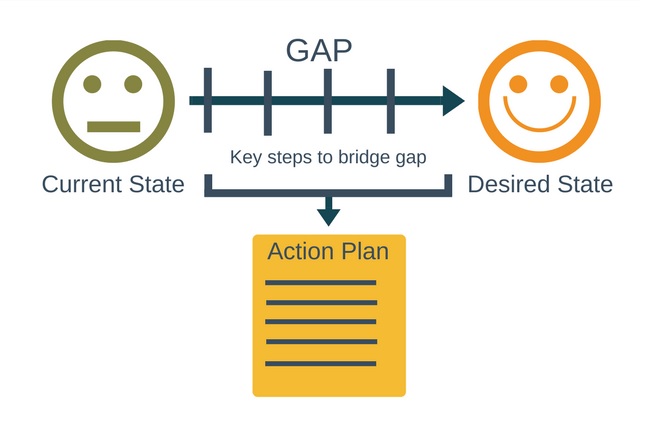
A gap analysis is a method of assessing the performance of a business unit to determine whether business requirements or objectives are being met and, if not, what steps to be taken to meet them. A gap analysis may also be referred to as a needs analysis, needs assessment or need-gap analysis.
We are leading pharmaceutical GMP audit consulting firm in APAC. PHARMEXCEL are planning to keep footprint globally in PAHRMA AND BIOTECHNOLOGY .
The company is involved in providing GMP auditing services such as , GAP assessment in the pharmaceutical industry, GAP analysis and GMP facility assessment, and GAP assessment in pharma industry, etc. GAP analysis is a way to compare current process and practices in order to identify gaps and areas in need of improvement with regards to compliance with the relevant standards, Gap analysis/ is used by all the pharmaceutical companies to analyze processes of any division of their company. In this process, we follow all the guidelines of all current practices and make a review for adequacy, suitability, sustainability, effectiveness, compliance.
Internal communications methodology , document control process, record keeping process, training process, internal audit process, management review process, measuring and monitoring process, non conformance management process, and continual improvement process.
In view of this intensive gap analysis/specialized evaluation of the documents/data/information empowers the pharma manufacturers to distinguish and moderate the gaps before regulatory accommodation.
This will keep away from Regulatory dismissals of the entries and limit the major/basic inquiries to attach the endorsements.
Gap analysis of the legacy dossiers against current practices followed in the manufacture of the medicinal products.
