WHAT IS Supply chain management and warehousing Goods Distribution practices ?
Supply chain management and warehousing Goods Distribution practices
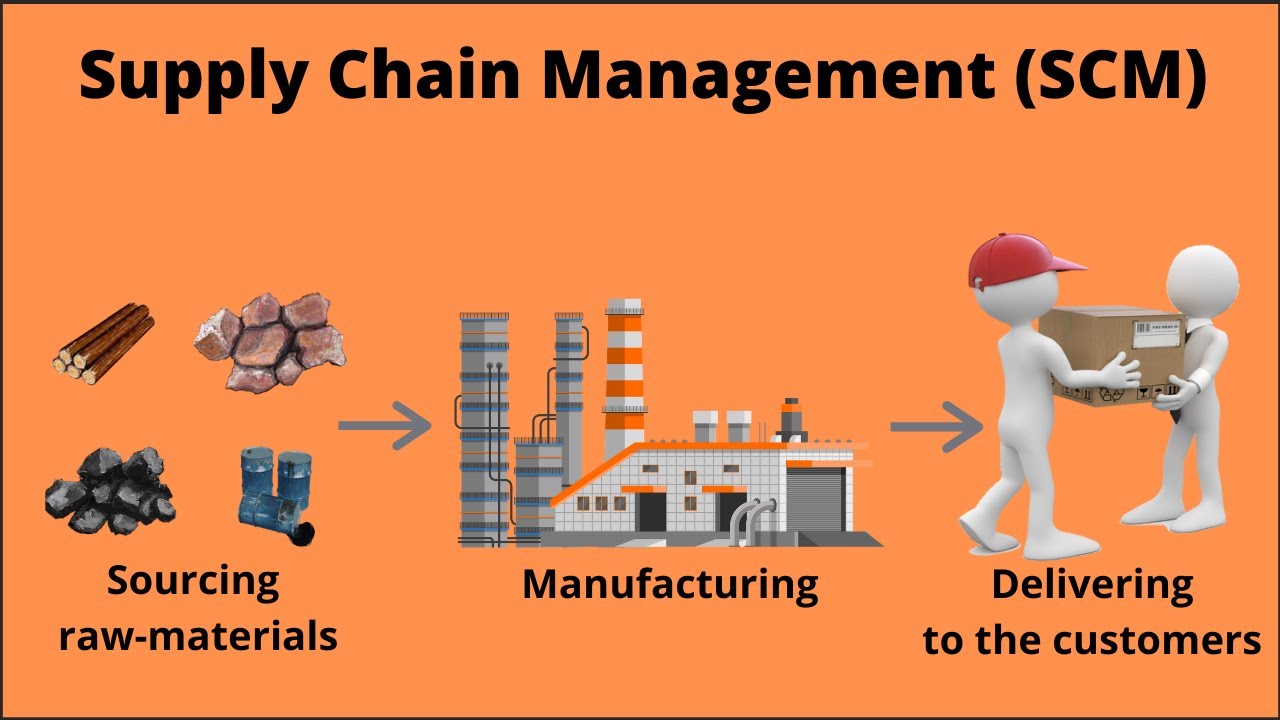
Supply chain management is the management of the flow of goods and services and includes all processes that transform raw materials into final products. It involves the active streamlining of a business’s supply-side activities to maximize customer value and gain a competitive advantage in the marketplace.
The basic steps of supply chain management-
The Top-level of this model has five different processes which are also known as components of Supply Chain Management – Plan, Source, Make, Deliver and Return.
Components of supply chain management-
Supply chains are composed of four major elements: procurement, operations, distribution, and integration. Supply chain management should not be seen as appropriate only for large businesses.
Supply chain management involves five main functions: aligning flows, integrating functions, coordinating processes, designing complex systems, and managing resources
The roles and responsibilities of supply chain.
Duties and responsibilities in a Supply Chain job
- Planning delivery timetables.
- Ensuring stores have enough stock.
- Making sure suppliers have enough stock to meet demand.
- Overseeing the ordering and packaging process.
- Monitoring stock levels.
- Tracking products through depots to make sure they arrive at their destination.
Key performance indicators (KPIs)
It is a set of quantitative metrics that can help you gauge your business’ performance over time. Specifically, they enable you to monitor how effectively your organization is achieving its target goals
Pharmaceutical supply chain solutions; GxP and GDP compliant; meeting all regulatory needs. Contact us for pharmacy warehousing services that ensure the security of your
Pharmaceutical Warehousing
Pharmaceuticals are stringently regulated by the Food and Drug Administration (FDA). The FDA’s regulatory standards for the industry are referred to as Current Good Manufacturing Practice (CGMPs; also known as “Good Manufacturing Practice” [GMP]) standards. These standards apply to warehousing processes, and to the drugs themselves.
- Annex 5 WHO good distribution practices for pharmaceutical products
CGMPs related to warehousing include:
- Drugs must be stored to prevent contamination, and be positioned to allow for inspection and cleaning of the area.
- Each lot of drug products must be identified with a distinctive (and traceable) code, and the lot’s status must be identified (approved, quarantined, rejected).
- Written procedures must describe the distribution process for each drug. This includes procedures for recalls.
- Written procedures must describe the appropriate storage conditions for each drug.
- Temperature-controlled space requires sophisticated control and monitoring equipment to ensure that the temperature of the facility stays within very specific parameters.
- Climate-controlled space regulates and monitors both the temperature and humidity of the space.
